
From 1850 until 2022, the ocean has absorbed 26 % of total anthropogenic emissions. Over the historical period, the ocean sink increased in pace with the exponential anthropogenic emissions increase. Ĭumulated since 1850, the ocean sink holds up to 175 ± 35 gigatons of carbon, with more than two-thirds of this amount (120 GtC) being taken up by the global ocean since 1960. However, the additional CO 2 in the ocean results in a wholesale shift in seawater acid-base chemistry toward more acidic, lower pH conditions and lower saturation states for carbonate minerals used in many marine organism shells and skeletons. The ocean acts as a carbon sink for anthropogenic CO 2 and takes up roughly a quarter of total anthropogenic CO 2 emissions. The source for this excess CO 2 is clearly established as human driven, reflecting a mix of anthropogenic fossil fuel, industrial, and land-use/land-change emissions. The current elevated levels and rapid growth rates are unprecedented in the past 55 million years of the geological record. Present-day (2021) atmospheric carbon dioxide (CO 2) levels of around 415 ppm are around 50% higher than preindustrial concentrations. The resulting ecological collapse in the oceans had long-lasting effects on the global carbon cycle and climate.
Ocean acidification has occurred previously in Earth's history. These strategies are being researched but generally have a low technology readiness level and many risks.IPCC (2022) Chapter 12: Cross sectoral perspectives Archived 13 October 2022 at the Wayback Machine in Climate Change 2022: Mitigation of Climate ChangeContribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change] Archived 2 August 2022 at the Wayback Machine, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA : 12–36 ocean alkalinity enhancement, enhanced weathering) could also reduce ocean acidification. The more specific ocean-based mitigation methods (e.g. Mitigation measures which achieve carbon dioxide removal from the atmosphere would help to reverse ocean acidification. climate change mitigation measures) is the only solution that addresses the root cause of ocean acidification. Reducing carbon dioxide emissions|url= (i.e. The United Nations Sustainable Development Goal 14 ("Life below Water") has a target to "minimize and address the impacts of ocean acidification".
Ongoing acidification of the oceans may therefore threaten food chains linked with the oceans. Some 1 billion people are wholly or partially dependent on the fishing, tourism, and coastal management services provided by coral reefs. The effects of ocean acidification are therefore impacting marine ecosystems that provide food, livelihoods, and other ecosystem services for a large portion of humanity. These include reduced calcification, depressed metabolic rates, lowered immune responses, and reduced energy for basic functions such as reproduction. ĭecreased ocean pH has a range of potentially harmful effects for marine organisms.
Other factors that influence the atmosphere-ocean CO 2 exchange, and thus local ocean acidification, include: ocean currents and upwelling zones, proximity to large continental rivers, sea ice coverage, and atmospheric exchange with nitrogen and sulfur from fossil fuel burning and agriculture. This can cause acidity to rise, lowering the pH and carbonate saturation levels in these areas. Colder and higher latitude waters are capable of absorbing more CO 2. Sea-surface pH and carbonate saturation states vary depending on ocean depth and location. Ī change in pH by 0.1 represents a 26% increase in hydrogen ion concentration in the world's oceans (the pH scale is logarithmic, so a change of one in pH unit is equivalent to a tenfold change in hydrogen ion concentration). Marine calcifying organisms, such as mollusks and corals, are especially vulnerable because they rely on calcium carbonate to build shells and skeletons.
CARBONIC ACID PRECIPITATE EQUATION FREE
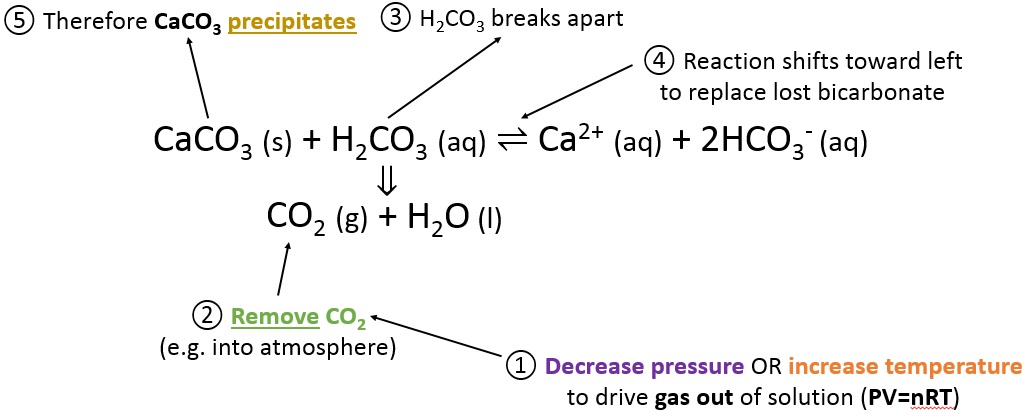
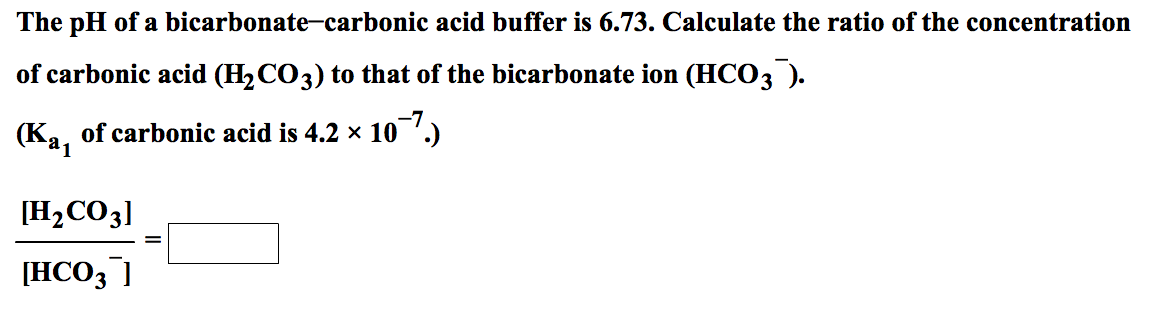
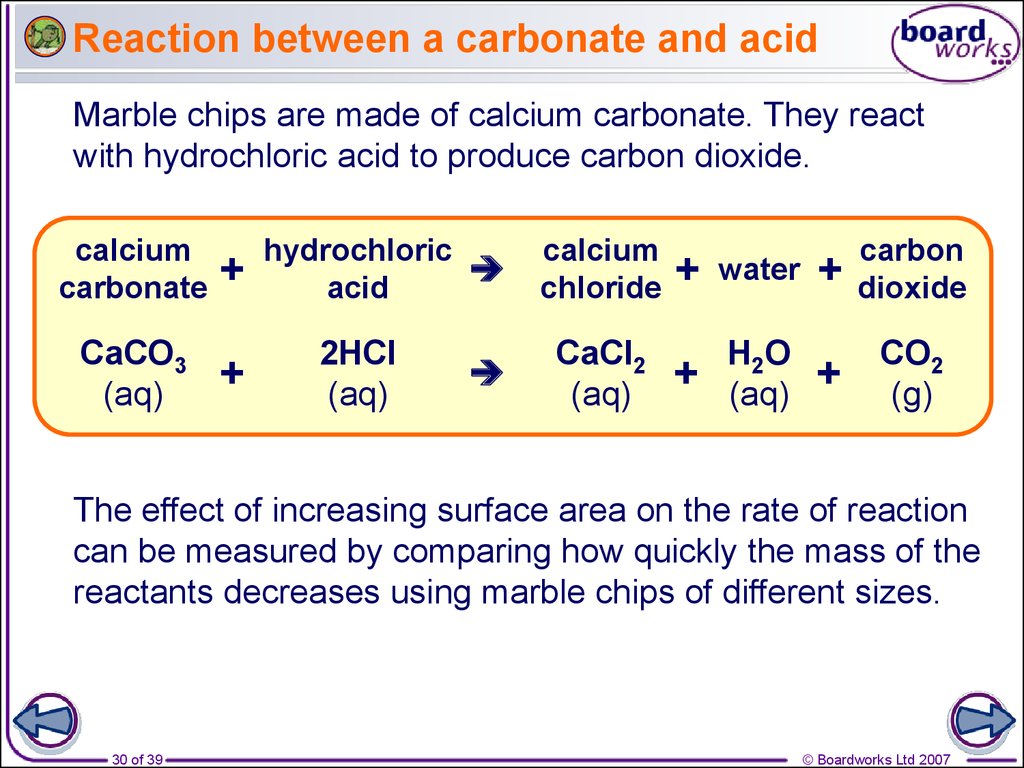
The presence of free hydrogen ions (H +) lowers the pH of the ocean, increasing acidity (this does not mean that seawater is acidic yet it is still alkaline, with a pH higher than 8). This produces carbonic acid (H 2CO 3) which dissociates into a bicarbonate ion ( HCO − 3) and a hydrogen ion (H +). CO 2 from the atmosphere is absorbed by the oceans. Carbon dioxide emissions from human activities are the primary cause of ocean acidification, with atmospheric carbon dioxide (CO 2) levels exceeding 410 ppm (in 2020). Between 19, the average pH of the ocean surface fell from approximately 8.15 to 8.05.
Ocean acidification is the decrease in the pH of the Earth's ocean. Climate change-induced decline of pH levels in the ocean Ocean acidification means that the average ocean pH value is dropping over time.


 0 kommentar(er)
0 kommentar(er)
